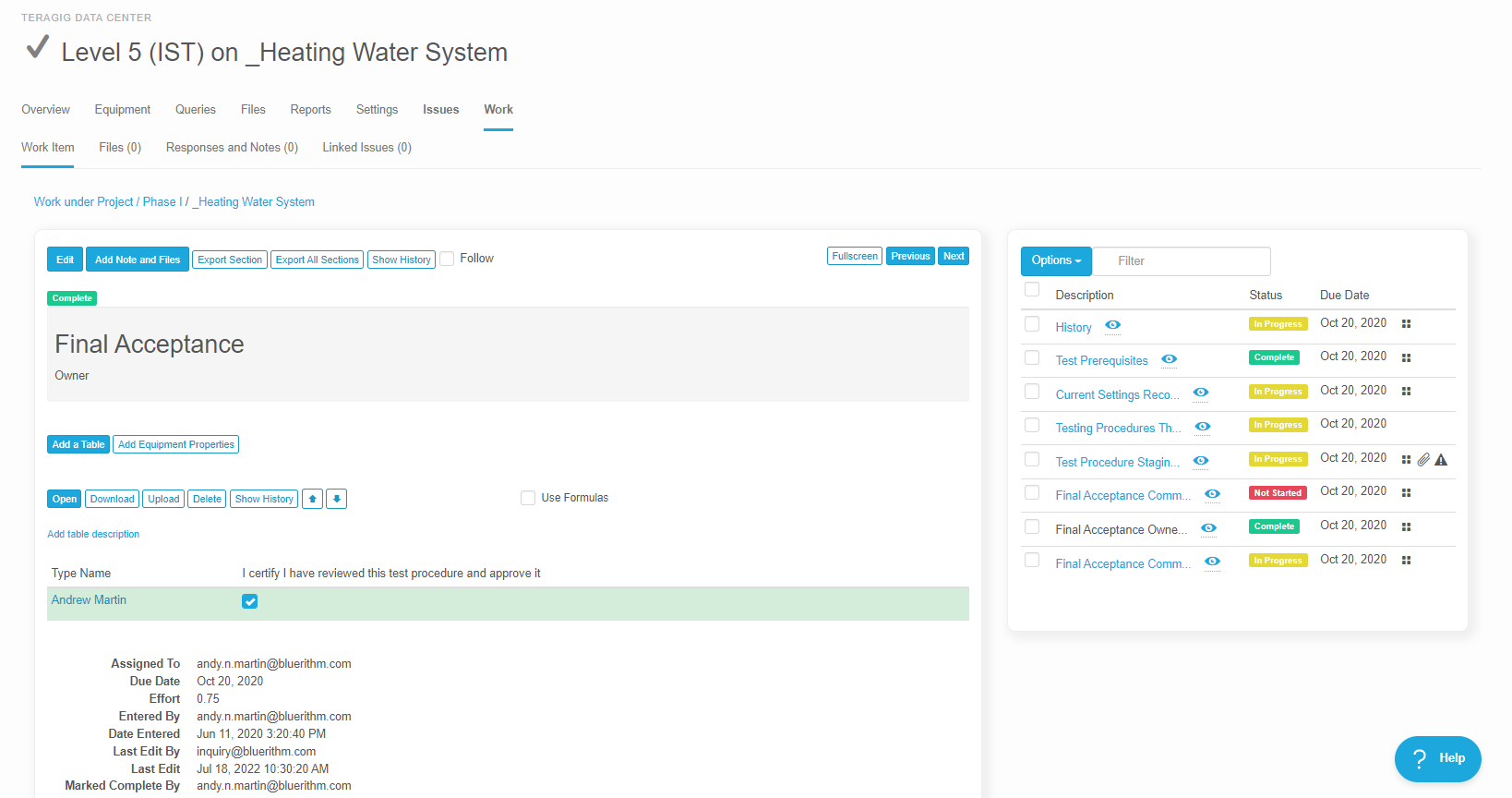
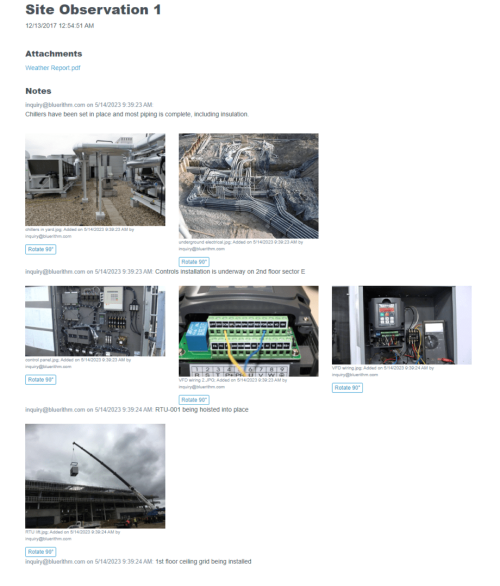
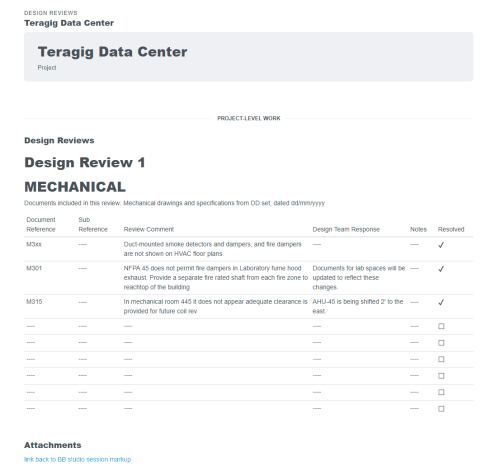
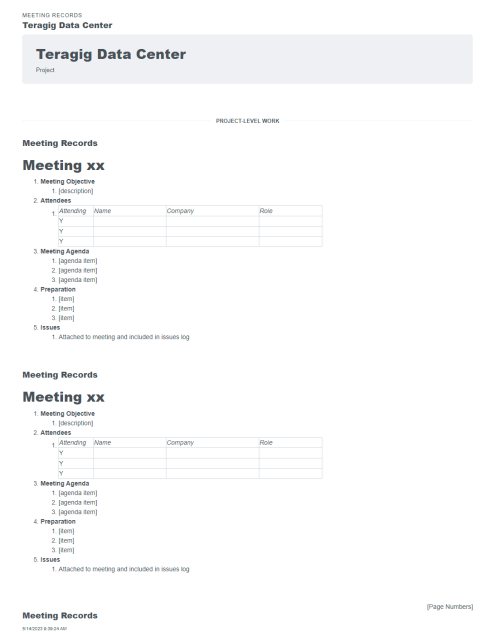
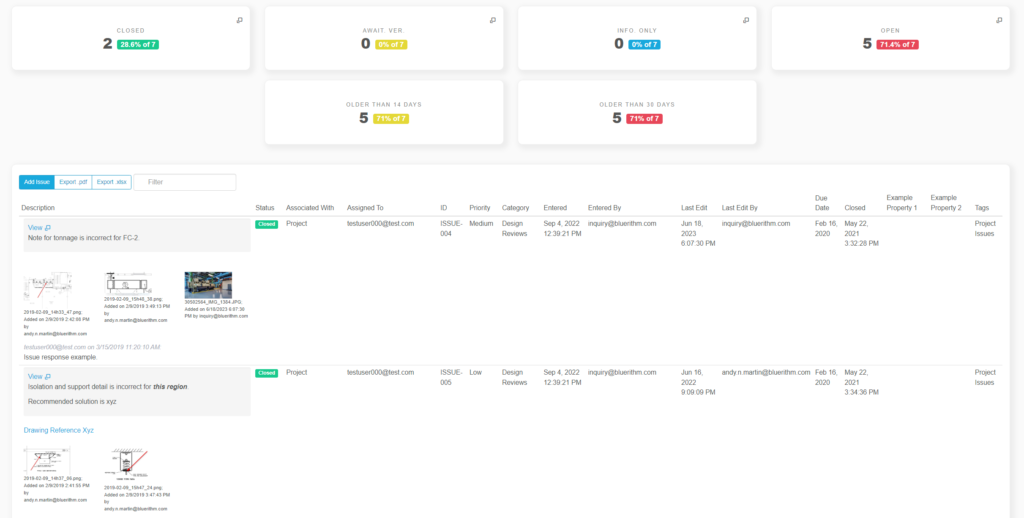
Some workflows and situations require approvals and sign-offs, often multiple levels of approvals. For example, 21 CFR Part 11 Compliance workflows for GMP facilities in the biopharma industry. Or several rounds of layers of review after a checksheet or test form has … Read More
Daily Archives: September 5, 2022
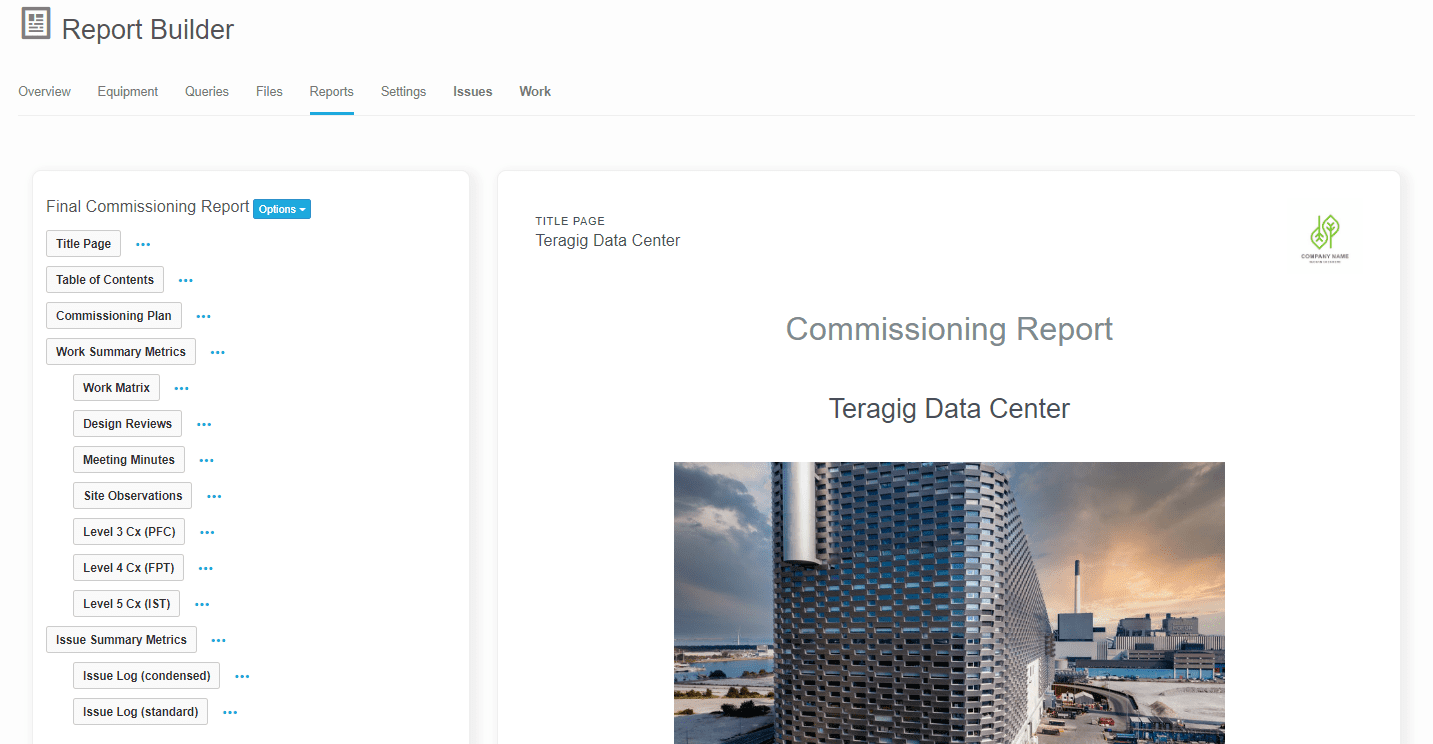
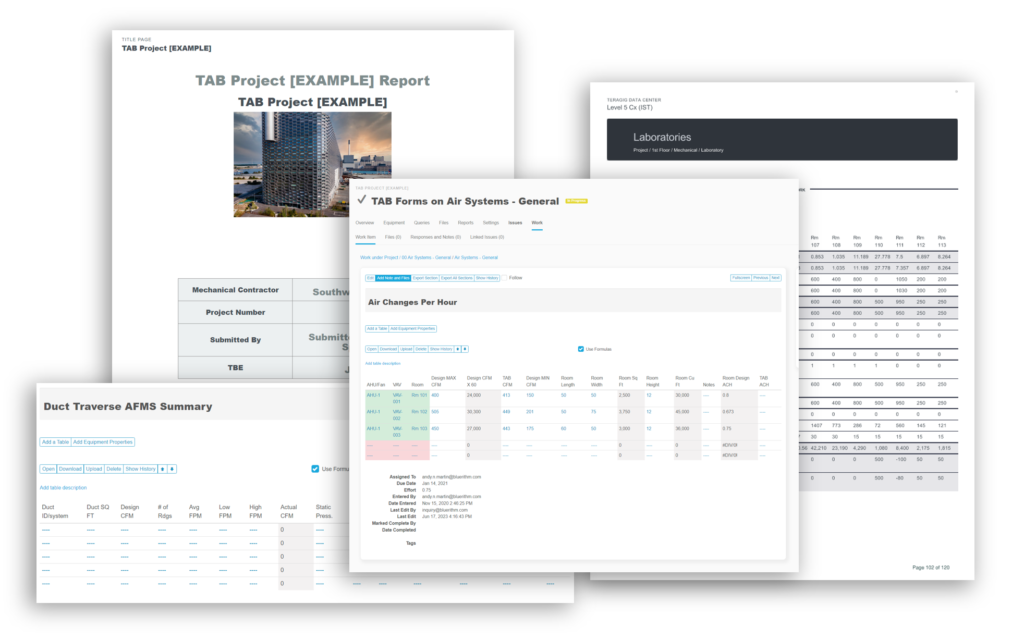
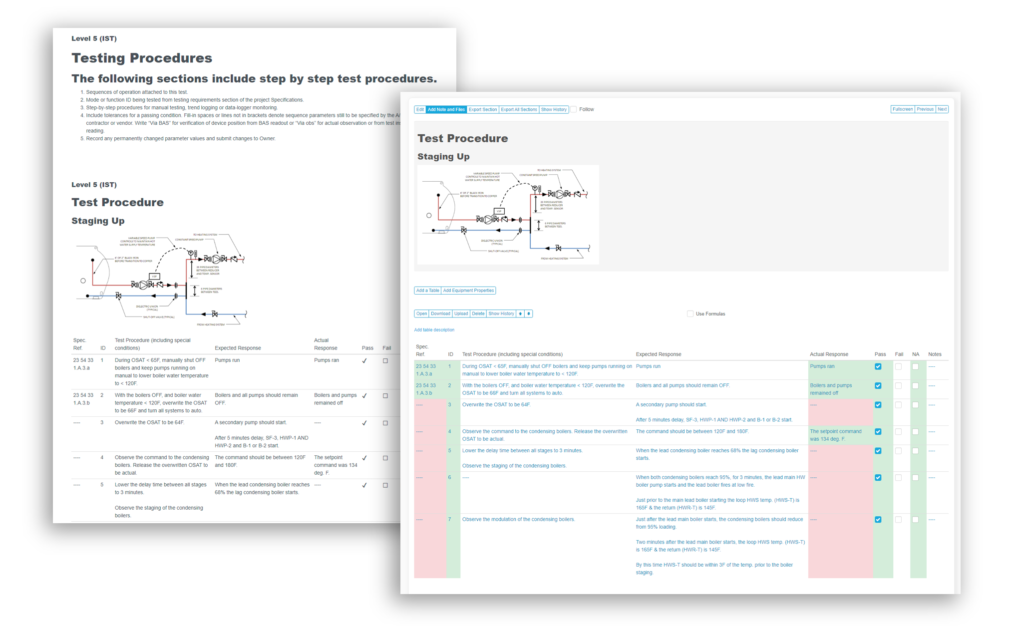
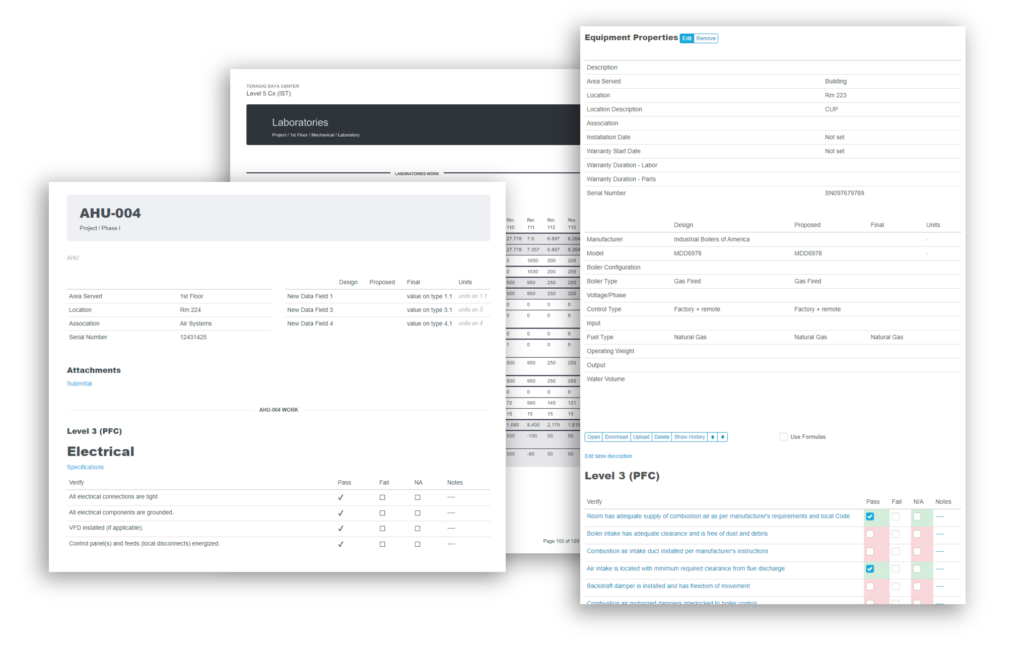
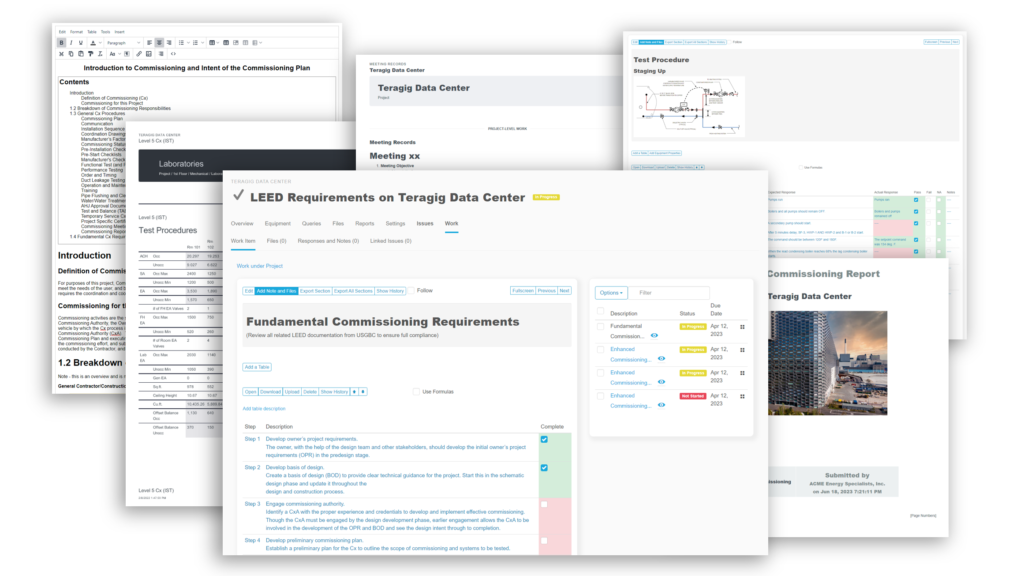
Bluerithm PDF Report Builder
In Bluerithm, there are many ways to build reports and extract data. There are, of course real-time data in pages to view the state of a project, the state of issues and punch list information, schedule tools, equipment lists, equipment … Read More