Software for Pharmaceutical Commissioning and Validation
For managing the rigors of commissioning and qualification of pharmaceutical, bio-tech, and medical device facility design and construction.
Play Video about Bluerithm demo
Automate Complexity
Pharmaceutical Commissioning and Qualification
Manage all commissioning and qualification efforts of a pharmaceutical manufacturing facility project.
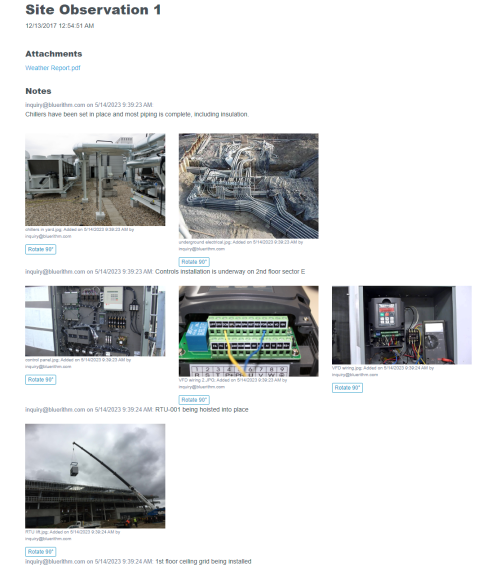
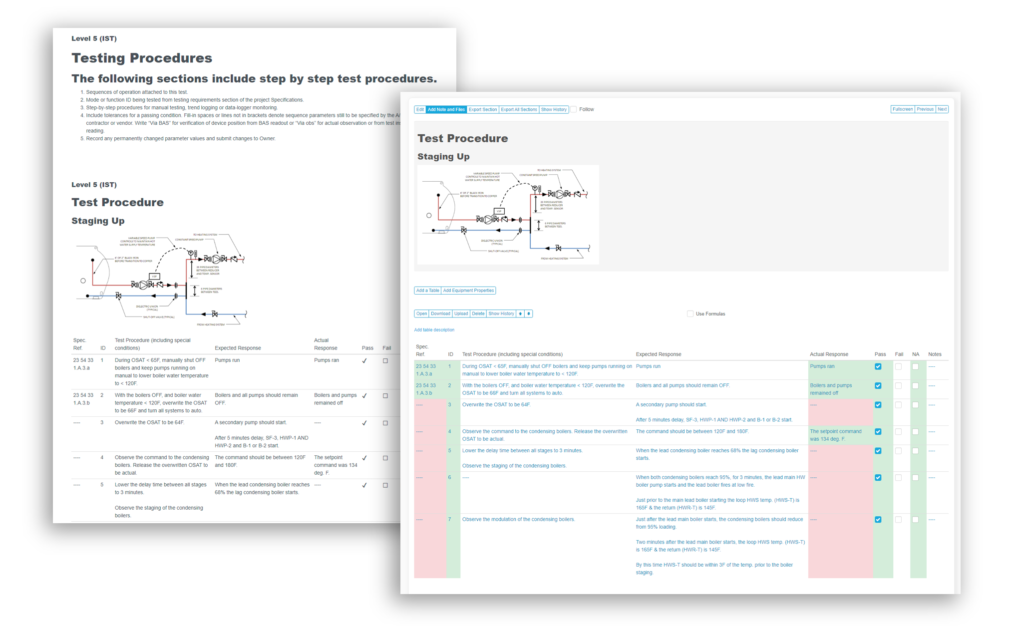
Automate reporting of documented evidence for the entire project.
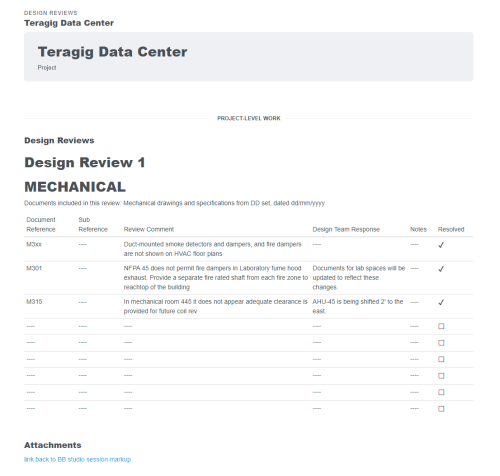
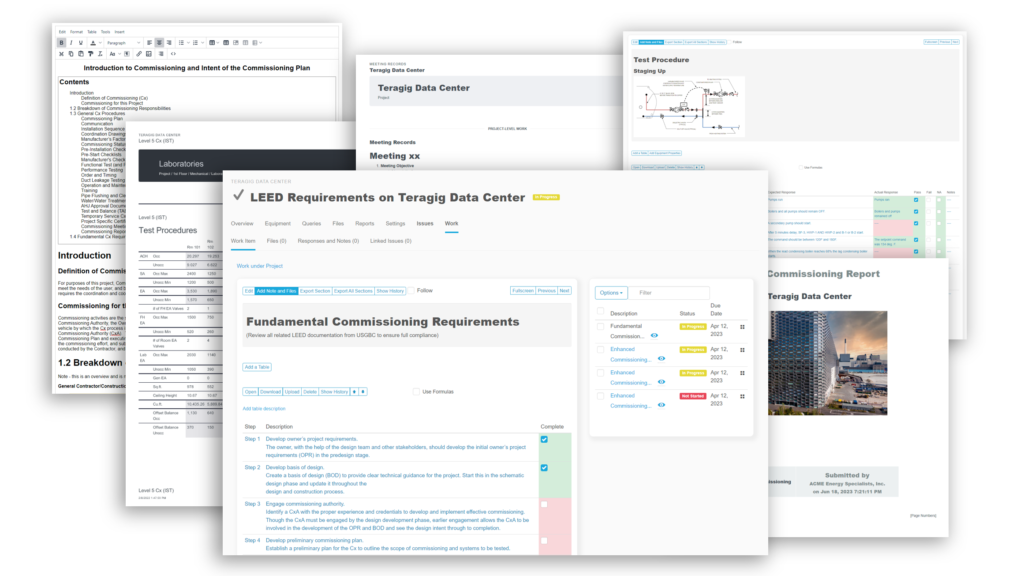
- CUSTOM workflows and forms based on your requirements. Easily added into templates
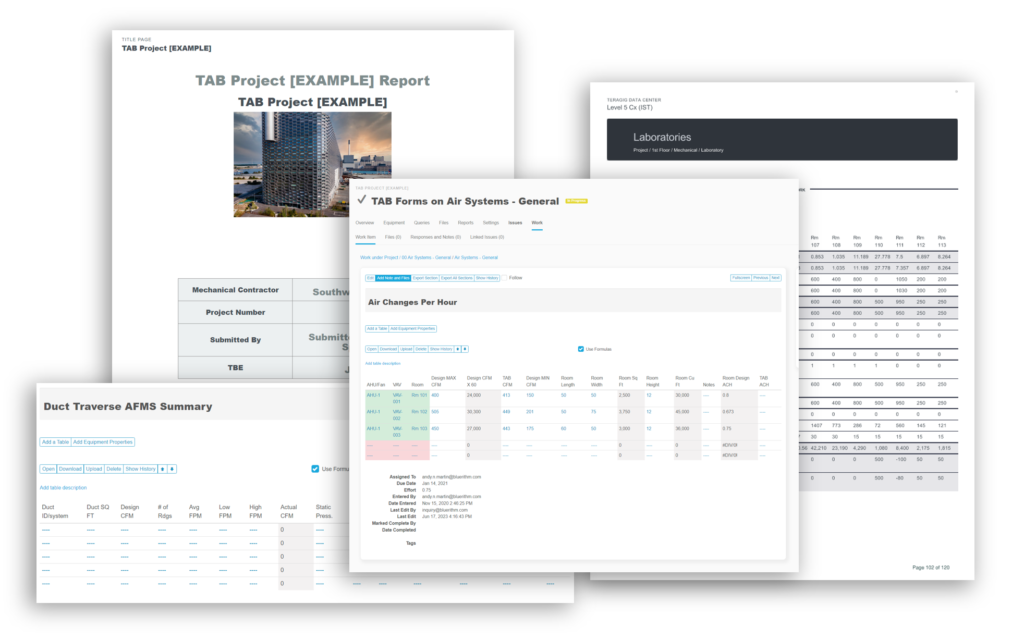
- Track and manage qualification processes: Design, installation, operational, and process performance
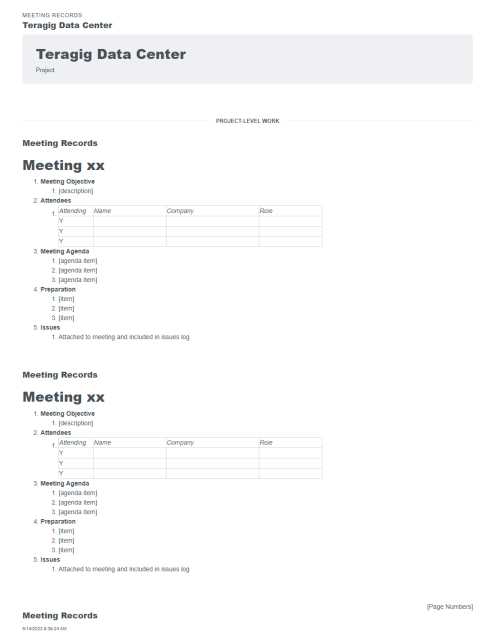
- Give all stakeholders (owner, engineering, quality control, suppliers) real-time, interactive access to all project data
- Traceability of all phases of commissioning and qualification
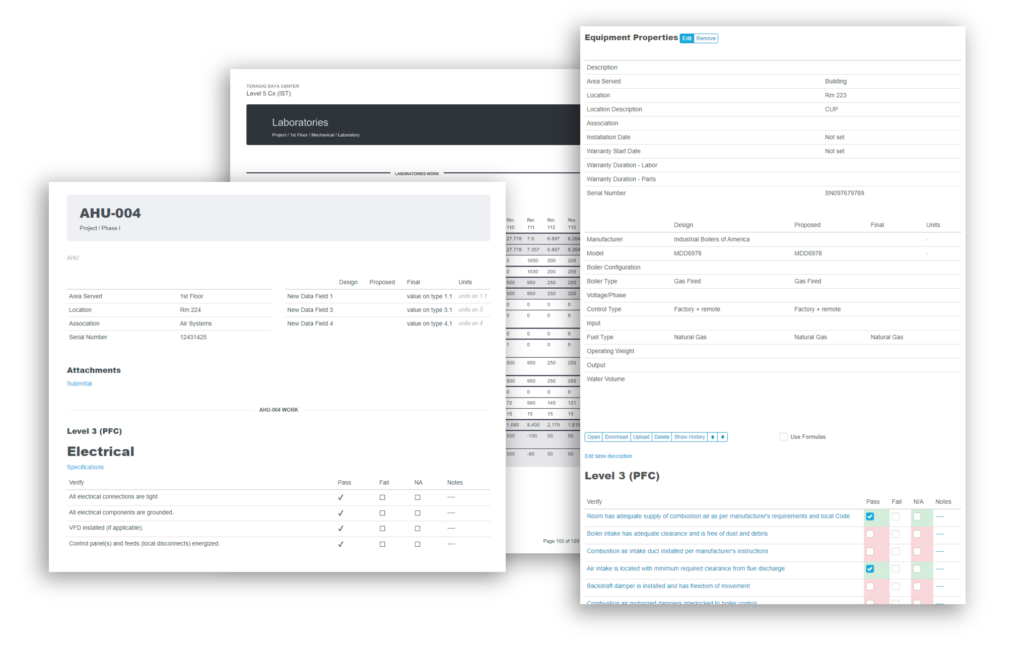
- Asset lists with custom tagging
- Build commonly used forms into templates that you, or we can help develop
- Input field data with Android, iOS, Windows, and web apps that work on, or offline
- Report automation (download a sample report)
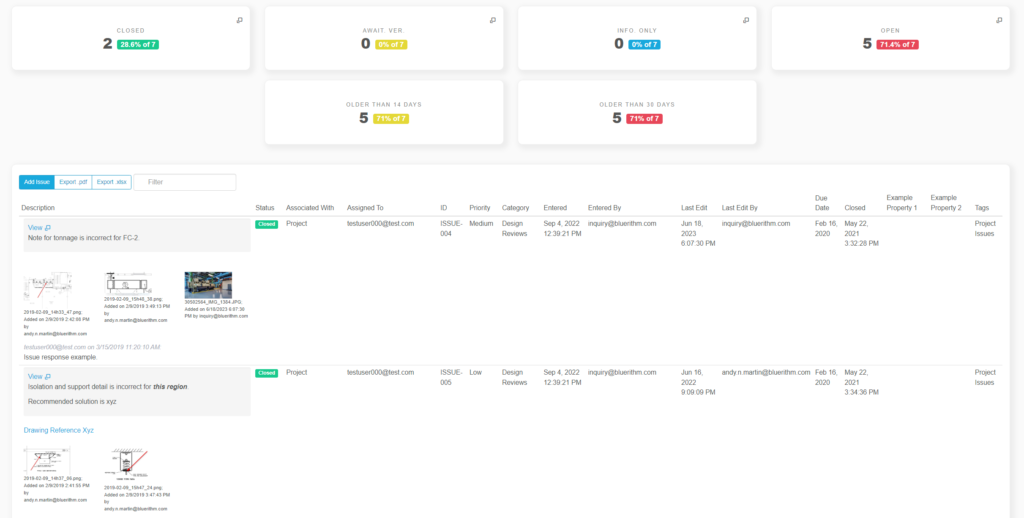
- Issue & punch list management. Collaborate in the cloud for high velocity communication and resolution
- Automated schedules (Gantt charts)
- Cloud-based collaboration
- Dashboards for you and your clients
- Import and export spreadsheets for super fast setup and editing
Streamline your projects
Start saving time and money today